Introduction: Diffuse large B-cell lymphoma (DLBCL) is featured by high heterogeneity, especially in clinical outcome. Tumor microenvironment (TME) represents a complex ecosystem around tumor cells, which has been found to be associated with the prognosis of DLBCL patients. However, the overall profile of TME immune components, perception of its correlation with tumor development and molecular mechanism are still insufficient in DLBCL. Here, we aimed to investigate novel prognostic scoring system and biomarkers based on TME immune components, and explore their underlying molecular mechanism in the development of DLBCL.
Methods: Microarray transcriptomic data and corresponding clinical information of DLBCL patients (n=946) from GSE23501, GSE53786, GSE10846, GSE136971, GSE57611 and GSE32918 in GEO database were analyzed. The immune composition was calculated by CIBERSORT. Immunohistochemistry (IHC) was used to assess the expression level of CD2 in DLBCL tissues. Seurat in R was employed for conducting single-cell analysis. The study was performed with the approval of the Medical Ethical Committee of Shandong Provincial Hospital, and all samples were collected with informed consents.
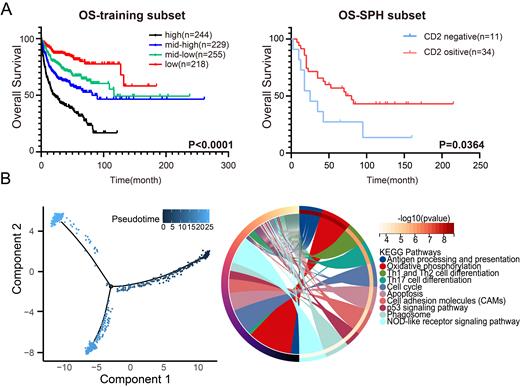
Results: To assess the immune cell composition of TME in DLBCL patients, we conducted a comprehensive analysis of the relative proportions of 22 distinct immune cell types within DLBCL TME. Subsequently, the prognostic role of TME immune components in DLBCL patients was probed, and a novel TME immune score based on the proportion of 22 immune cells was established. Furthermore, to evaluate the association between TME immune score and clinical prognosis in DLBCL patients, we performed K-M analysis and 3,5,10-year ROC analysis, which revealed the high prognostic efficacy of TME immune score. Higher TME immune scores were demonstrated to be significantly associated with worse prognosis, which was subsequently validated in an external cohort containing 230 DLBCL patients. Survival data of quartile subgroups also unveiled the prognostic value of TME immune score in DLBCL patients ( Fig. 1A).
We further explored the correlation between TME immune score and clinical pathological features in DLBCL patients. Higher TME immune scores were observed to be correlated with more advanced Ann Arbor stage, non-germinal center B-cell-like subtype, lower expression levels of immune checkpoint molecules (CTLA-4, PD-1, PD-L2), decreased ESTIMATE stromal and immune scores, as well as higher tumor purity, indicating that the TME immune score may enable the prediction of treatment response to immune checkpoint inhibitors.
The potential molecular mechanism of TME modulation in DLBCL development was further explored. Results of GO and KEGG enrichment illuminated that differential expression genes (DEGs) in two groups were significantly enriched in the anti-tumor immunity related processes and pathways, especially T-cell receptor activation. Subsequently, 6 hub genes (CCL5, CD2, CD3E, MMP9, CCR5, GATA3) in protein-protein interaction network constructed by DEGs were identified, whose expression levels were found to be associated with prognosis in DLBCL patients. Moreover, IHC was used to investigate the relevance of CD2 protein expression with prognosis in DLBCL patients. The expression of CD2 protein was found to be associated with poor prognosis in DLBCL patients ( Fig. 1A).
To further investigate the regulatory function of CD2 in the immune microenvironment of DLBCL, we conducted single-cell analysis based on three DLBCL patients, and revealed that CD2 was prominently expressed in T cell subpopulation and was correlated with different differentiation trajectory of T cells in DLBCL. Subsequent differential gene analysis revealed that DEGs between state 1 and state 2, 3 were significantly enriched in KEGG pathways including antigen presentation and Th cell differentiation ( Fig. 1B), suggesting the role of CD2 in T cell activation and differentiation.
Conclusions: Taken together, we established a novel TME immune score which could serve as a prognostic indicator in DLBCL patients. More importantly, we identified CD2 as a prognostic biomarker in DLBCL patients and unveiled its association with different T-cell activation states. Our study might provide a novel prognostic stratification strategy and promising biomarkers, which could facilitate the individualized management in DLBCL patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal